BMGF reference: OPP1155293
The TRYP-ELIM-BANDUNDU project is a BMGF investment led by the Institute of Tropical Medicine in Antwerp (ITM), Belgium and includes, as partners, the national HAT control programme of DRC (PNLTHA), PATH and the Liverpool School of Tropical Medicine (LSTM). The consortium is working in the former Bandundu province (now comprising Kwilu, Kwango and Mai-Ndombe Provinces) in DRC to implement a substantial expansion of gHAT control activities. In recent years up to 90% of global gHAT cases have been reported in DRC and within the country, in 2014 ~58% of DRC gHAT cases were from Bandundu making this area the major contributor to the global HAT burden.
ITM and PNLTHA, are working to scale-up active and passive screening capacity for gHAT using innovative approaches developed in previous grants. LSTM is working with PNLTHA to implement a huge expansion of tsetse control using Tiny Targets in areas where screening alone is predicted not to achieve the elimination goals. As a consortium we are developing of an integrated data management system. PATH provide logistical support for all components of the project.
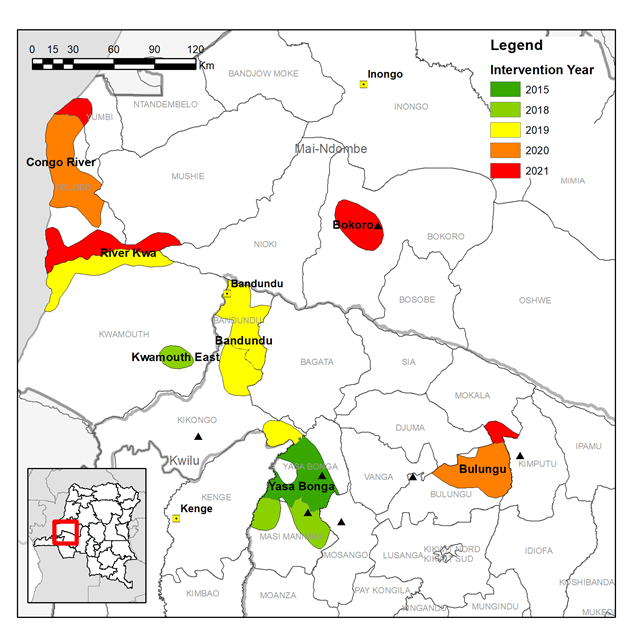
LSTM and PNLTHA have previously implemented tsetse control over ~3,000 km2 of Yasa Bonga, Masi Manimba and Kwamouth Health Zones, all part of the former Bandundu province, in a project designed and managed largely by LSTM. In TRYP-ELIM-BANDUNDU we will transition to a large-scale and sustainable program led by PNLTHA covering ~11,700 km2 in 11 Health Zones. The transition requires not only a four-fold increase in the tsetse control intervention area but also (i) an expansion of national, provincial and local capacity to design, implement and monitor tsetse control, (ii) raise awareness of the benefits of tsetse control at national and local levels and (iii) develop a data management system which integrates data from screening and vector control programs. To achieve this transition and successfully implement tsetse control at scale we have developed four work packages:
1. Expand tsetse control operations to 11 priority Health Zones adapting the current Tiny Target deployment model from a LSTM-directed to a PNLTHA-directed one. (Consortium lead: PNLTHA, supported by LSTM).
Based on the distribution of HAT cases we identified fourteen priority intervention areas across eleven Health Zones where tsetse control will accelerate progress towards the local elimination of HAT as a public health problem.
The expansion of vector control will be done in a phased manner over the course of the project. Intervention areas will have two rounds of Tiny Target deployment per year, this will be done by local teams largely operating from traditional dug-out canoes, “pirogues”, on main rivers. Entomological surveys will measure the impact of the intervention on the tsetse population.
2. Build and strengthen the capacity of PNLTHA to plan and manage tsetse control operations at full country scale (Consortium lead: LSTM).
Historically tsetse control in DRC was done using traps deployed by screening teams but due to costs this activity was not implemented systematically at scale. Previously, there were only two people dedicated to vector control within PNLTHA, both based in Kinshasa. To implement at scale, an appropriate vector control structure is required with staff involved at all levels (central, provincial and Health Zone).
Capacity strengthening of people and systems is critical for the long-term sustainability of vector control in DRC. We are working with the CCR at LSTM to implement their 5-step methodology for designing and evaluating our capacity strengthening programme.
3. Community involvement in vector control activities in HAT-affected Health Zones (Consortium lead: ITM).
ITM lead a work package on community involvement in tsetse control. There are two main approaches, the first is the implementation of the vector control sensitization strategy, the second uses a community-based model for the deployment of Tiny Targets. The sensitization will involve working with the central and provincial levels of the PNLTHA and the Health Zones to use the traditional networks for sensitization to target all affected communities. The community-based deployment will be done in selected villages in hard to reach areas away from the main rivers where Tiny Targets are deployed in Work Package 1. This work package is complementary to WP1 and will help to ensure community acceptance of tsetse control.
4. Establish an integrated strategy to eliminate HAT by strengthening systems to plan interventions using integrated data management systems (Consortium lead: ITM).
Currently data management of medical and vector control activities is carried out largely independently of each other. Under a previous BMGF investment ITM developed a data management system for medical data collected during screening including a web-based information and decision support system. In TRYP-ELIMBANDUNDU we will use this system as the basis for integrating vector control data.
Improving the data linkage and communication between the “medical” and the “entomological” arms of the control program will improve the efficiency of our efforts against HAT.
The TRYP-ELIM-BANDUNDU investment is implementing an integrated medical and vector control programme using innovative tools at large-scale in the gHAT focus that contributes the majority of global cases in an effort to reach gHAT elimination.






