The Liverpool Vaccine Group

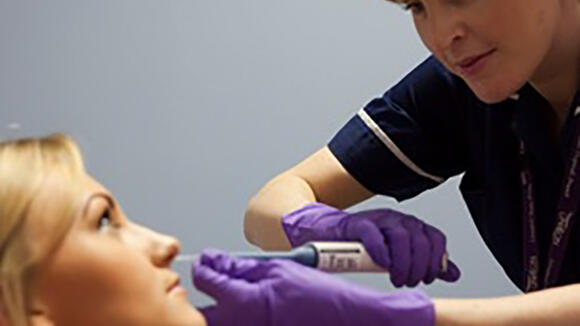
The Experimental Human Pneumococcal Carriage (EHPC) Consortium, funded for 5 years by the Medical Research Council (MRC), will make use of studies in healthy volunteers and patients with increased risk of pneumococcal disease to understand why some people are protected against pneumococcal carriage and others are not. We have developed a method, unique in the world, for inoculating humans safely with live bacteria in order to establish carriage experimentally and have now tested it in over 1000 subjects without adverse effects.
Testing new vaccines using EHPC can be done more quickly and at a fraction of the cost of clinical studies (100 subjects rather than many thousands) and so several vaccines can be tested during this Programme, in parallel with the discovery science. This Programme also offers an opportunity for partnership with commercial entities or charities sponsoring particular new vaccines and these funding options will be explored with MRC.
Participate in our clinical trials
Research at LSTM is working towards developing new vaccination in the prevention of pneumonia and without volunteers, progress in our research would be impossible.
- Find out the facts about pneumonia and how your participation could potentially benefit millions of people
- Everything you need to know about participating in a clinical trial, from start to finish
- You will be compensated for your time and participation
Find out more in our volunteers pages.
Accelerator Research Clinic (ARC)
A purpose built state of the art research clinic staffed by experienced doctors and nurses specifically trained in the EHPC model.