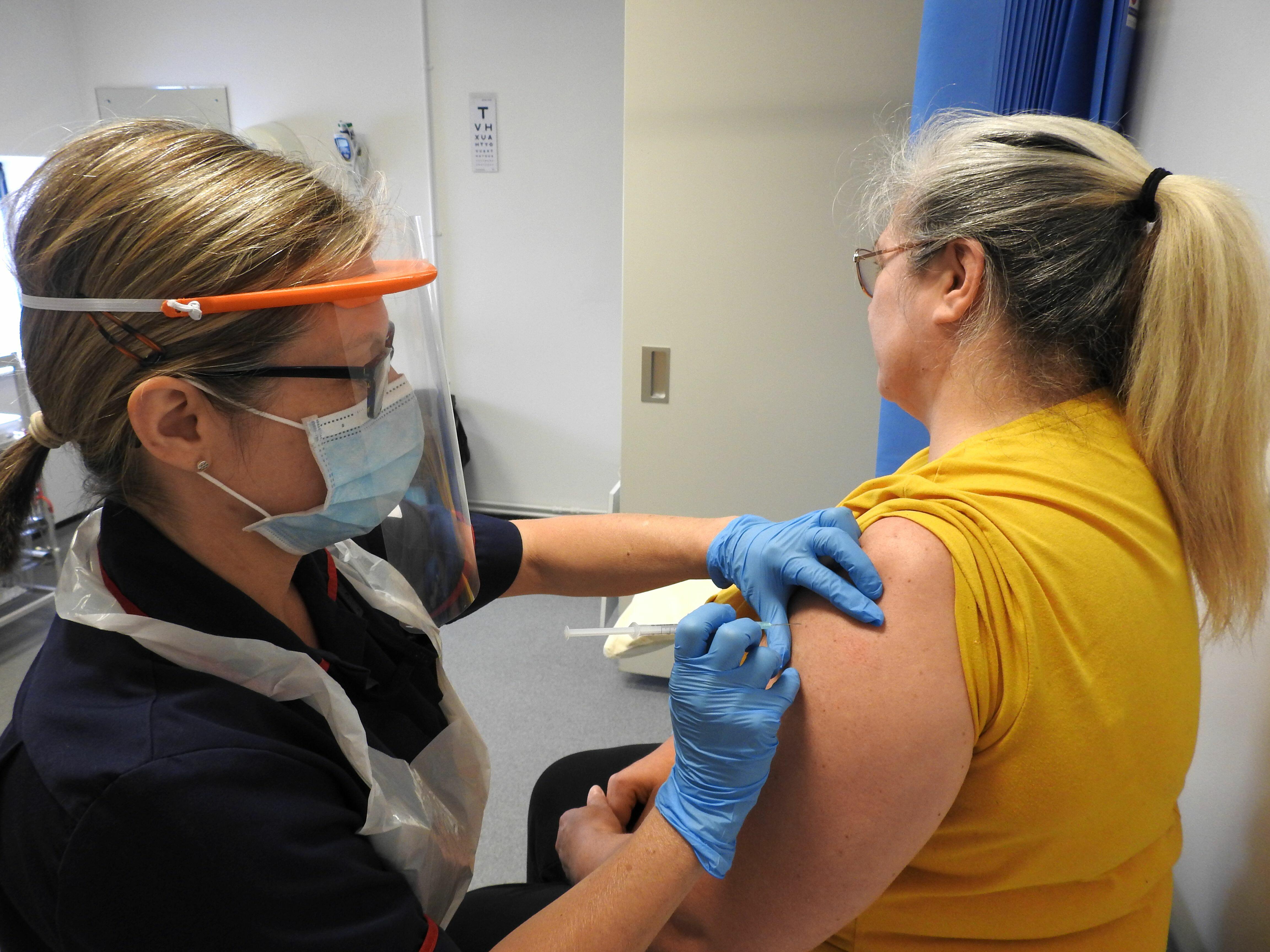
LSTM has completed its first week of vaccinating participants as part of the COVID-19 Oxford vaccine trial, for which LSTM is a phase III site.
Following a successful week of screening hundreds of potential participants, the activity moved to LSTM’s Well Travelled Clinic where more than 250 volunteers, mainly those working on the medical frontlines or in care or residential homes, were vaccinated. They received either the vaccine candidate developed at the University of Oxford, ChAdOx1 nCoV-19, or the Men ACWY meningitis vaccine in the randomised single blind study.
LSTM’s Respiratory Clinical Research Group, led by Professor Daniela Ferreira, are managing the trial at LSTM and have worked with countless volunteers as well as their own specialist research nurses and the specialist travel health nurses from the Well Travelled Clinic to screen and then vaccinate participants. Professor Ferreira said: “We have been blown away by the hard work of all those involved in the trial, not only from our many volunteers, who have provided their time and expertise for nothing, but all of those who have volunteered to come forward to become clinical participants in the trial and test the efficacy of this vaccine which, if effective, could save lives across the world.”
Phil Tubb is Managing Director of LSTM’s Well Travelled Clinics. She said: “We are delighted to be working with the Respiratory Clinical Research Group on this clinical trial. Our nurses specialise in vaccination, so we were happy to put that expertise to use working with our colleagues on such an important trial.”
The trial is continuing to recruit healthy adults aged between 18-55, with the remit widened to include all hospital staff and primary care staff and other key workers, while also beginning to recruit a cohort aged between 56-70 years from any profession, who are working (not from home) and in good health. If you would like to find out more or volunteer to take part, you will find more information here.