BBSRC project ref. BB/S01375X/1
Edges of wilderness areas are often ‘hotspots’ for disease, as pathogens naturally occurring in wild animals can spill over to infect people and livestock in surrounding areas, in often poor and marginalised populations. ENABLES builds on our work1 at the edge of the Serengeti National Park in Tanzania where tsetse flies transmit trypanosomes. These single-celled parasites do not cause any overt disease in wild animals, but do cause a disease called Nagana in livestock. Humans bitten by tsetse infected with a particular species of trypanosome can develop sleeping sickness (Rhodesian human African trypanosomiasis, r-HAT), an acute and fatal disease for which there is no preventative vaccine or drug. About 12 million people in east and southern Africa are at risk of r-HAT and the preservation of wilderness areas presents a chronic and intractable source of infection. Only Botswana has been able to solve the problem of r-HAT permanently by eradicating tsetse from the Okavango wilderness area through large-scale (~15,000 km2) aerial spraying. This complex and expensive solution is not feasible for most poor communities living on the edge of wilderness areas.
Our previous work1 in Serengeti suggests that livestock-keepers are preventing the spread of r-HAT by simply applying pyrethroid insecticides to their cattle. They do this to control the ticks and tsetse that carry livestock diseases (East Coast Fever, Nagana) but this cheap (~$6/animal/year) and easy method also interrupts transmission of r-HAT. By treating livestock to prevent animal diseases, livestock-keepers are protecting local people from r-HAT. Other countries have tried but failed to encourage widespread use of pyrethroids by livestock-keepers as part of a One Health approach against r-HAT. Uptake in Tanzania appears to be stimulated, at least in part, by a government subsidy on the price of pyrethroids.
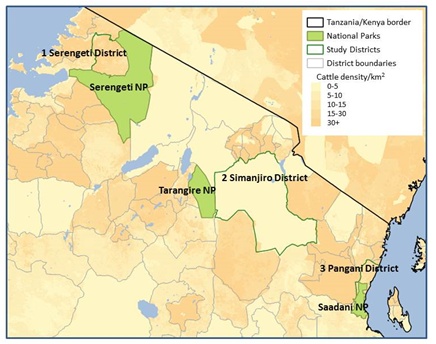
While our discoveries in Serengeti offer the exciting prospect of a One Health solution to r-HAT, we do not know (i) how widespread pyrethroid use is in different livestock systems, (ii) how generally applicable this approach is, and (iii) what conditions make farmer-led control likely and effective. ENABLES will test the hypothesis that national policies to promote use of pyrethroids by livestock-keepers have led to large-scale control of human and animal trypanosomiasis. To achieve this, we will first use satellite data to predict where tsetse are abundant, and then test the predictions by comparing the observed and expected number of tsetse caught in traps placed at various sites. Using a validated model of tsetse abundance, we will identify ‘hotspots’ where transmission is predicted to be high.
In selected ‘hotspots’ (Figure 1) from areas where different livestock systems (pastoralist, mixed crop-livestock, zero-grazing) predominate, we will test cattle for infection with trypanosomes and the presence of pyrethroids. We will conduct a questionnaire survey of herd owners about the decision-making underpinning methods used to control tsetse- and tick-borne diseases. We will also interview owners of veterinary outlets, national distributors of veterinary medicines and government officials to gain insights into the pyrethroid supply chain. Finally, we will use models to integrate data on the density of tsetse, prevalence of trypanosomes and use of pyrethroids to quantify the epidemiological benefit of pyrethroid-treated cattle. Empirical findings and models will form the basis of a toolbox to assist Tanzania and other countries to formulate evidence-based policies and practices for the sustainable control of sleeping sickness.
The ENABLES project is a collaboration of groups from the Liverpool School of Tropical Medicine (Steve Torr, Rachel Lea, Jennifer Lord, Isabel Saldanha, Michelle Stanton), the Vector and Vector-Borne Diseases Research Institute in Tanga, Tanzania (Oliva Manangwa), the Tanzanian Veterinary Laboratory Agency (Furaha Mramba), the University of Glasgow (Harriet Auty, Shauna Richards), the Roslin Institute at the University of Edinburgh (Liam Morrison) and economics consultant Alex Shaw.


