As part of the HUGS project, two newly published studies shed important light on Strongyloides fuelleborni, a parasitic worm historically associated with non-human primates but increasingly recognized for its zoonotic potential, that is, the ability to infect humans.
Vervet monkeys and zoonotic risk in Malawi
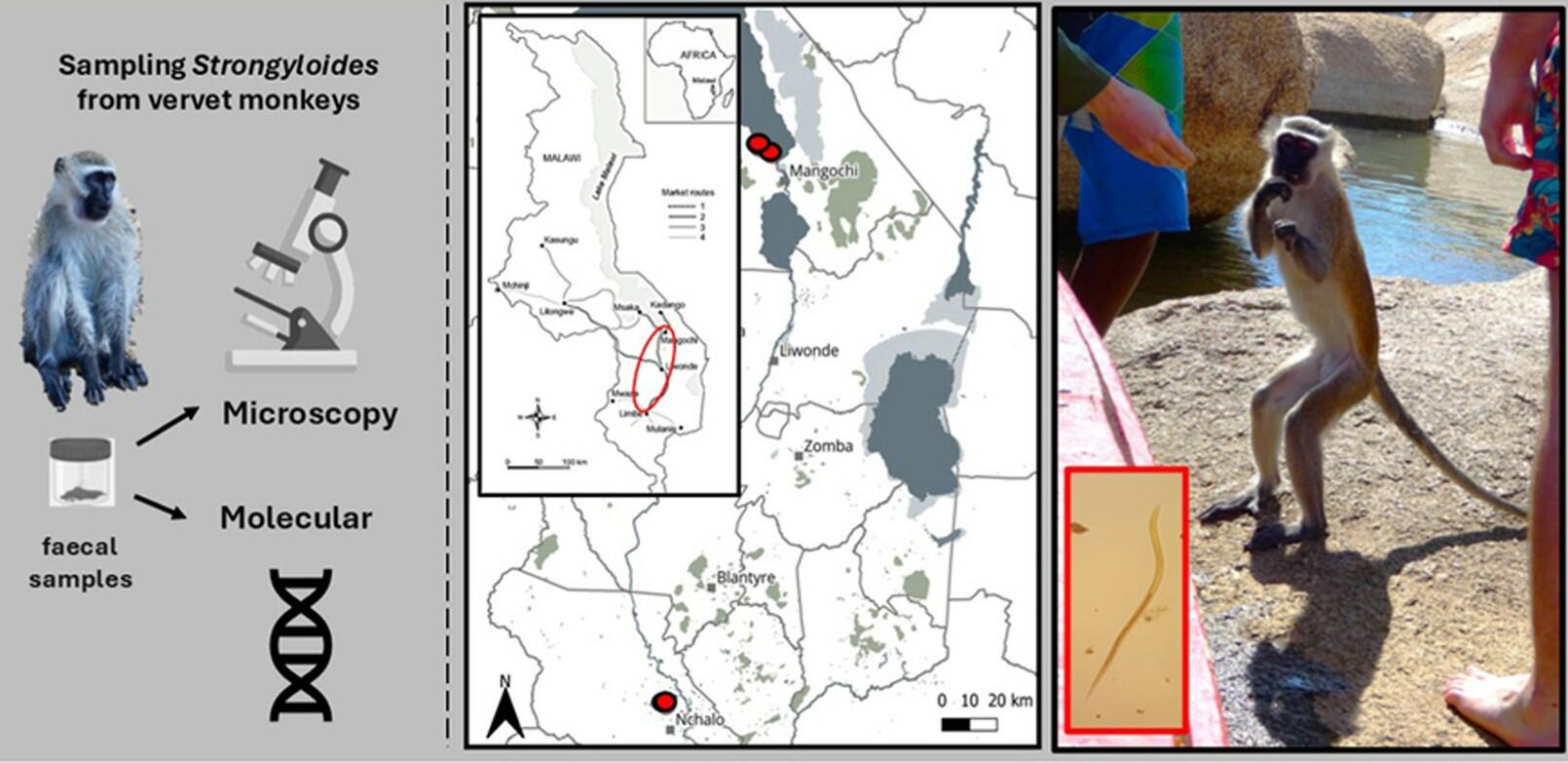
In the first study, we focused on vervet monkeys (Chlorocebus pygerythrus), an adaptable and opportunistic primate species that lives in close proximity to people across southern Malawi. Fieldwork was carried out in July 2023 in four locations, including a wildlife reserve within a sugarcane plantation and three lakeside tourist lodges. Thirty-two faecal samples were collected from monkey sleeping sites.
Using a combination of faecal culture, microscopy and molecular diagnostics, we detected S. fuelleborni larvae in three of the four locations. This marks the first molecular confirmation of the species in Malawi and links the vervet monkey more firmly as a potential reservoir host. Given that humans and monkeys often share these environments, and that people frequently walk barefoot, our findings reinforce the need to assess local zoonotic transmission risks more seriously.
Interestingly, this line of inquiry began when S. fuelleborni DNA was found in stool samples from schoolchildren during HUGS pilot surveillance. While the infection rate was low (~1.8%), it pointed to a non-sterile environmental reservoir, and vervet monkeys were an obvious suspect.
Mapping the genetics: an African–Asian divide
The second paper expands the story further by analysing the mitochondrial cox1 gene from S. fuelleborni larvae collected from vervet monkeys and comparing them to global reference samples. The results show a clear genetic division between African and Asian haplotypes, suggesting geographically distinct parasite lineages.
Our Malawian samples clustered with Tanzanian and Central African isolates, forming an “East African clade”. This supports previous work that shows S. fuelleborni in Africa is genetically different from populations found in Southeast Asia (e.g., in macaques) and raises the possibility that we are not dealing with a single species, but a species complex with cryptic diversity.
This distinction matters: understanding local genetic variation is key to developing diagnostic tools, interpreting pathogenic potential, and designing targeted interventions.
One Health but many hosts
Together, these two studies highlight the urgent need for integrated parasite surveillance, especially for under-researched helminths like Strongyloides. As human-wildlife interfaces expand due to deforestation, tourism, and agriculture, zoonotic infections can become more common and harder to track.
Papers:
- Juhász et al. (2025). Notes on the threadworm Strongyloides fuelleborni in vervet monkeys and zoonotic strongyloidiasis in southern Malawi. https://www.sciencedirect.com/science/article/pii/S2213224425000860?via%3Dihub
- Richins et al. (2025). Genetic diversity within Strongyloides fuelleborni: mitochondrial genome analysis reveals a clear African and Asian division https://www.cambridge.org/core/journals/parasitology/article/genetic-diversity-within-strongyloides-fuelleborni-mitochondrial-genome-analysis-reveals-a-clear-african-and-asian-division/6081AF32CB7D0DF4C7CF97ACB097AAAB?utm_campaign=shareaholic&utm_medium=copy_link&utm_source=bookmark.
Stay tuned for more zoonotic surprises!